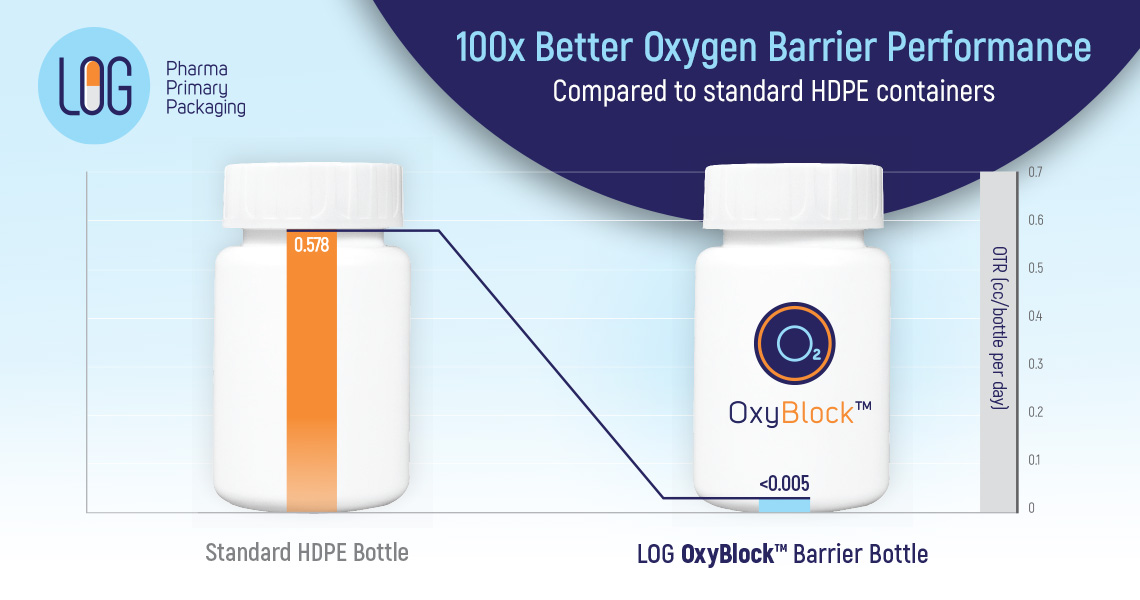
LOG’s OxyBlock® barrier packaging solution which is endorsed and used by global pharmaceutical companies for the last 10 years, offers outstanding barrier protection for formulations sensitive to oxygen such as Atorvastatin, Aspirin-Dipyridamole, etc…
- Based on OTR (Oxygen Transmission Rate) test results performed on 60ml bottles, OxyBlock® has a 100x greater oxygen barrier performance compared to standard HDPE containers.
- The OxyBlock® family is available in standard sizes from 40ml to 1500ml.
To learn more about OxyBlock® or to receive samples, Click here.
More about OxyBlock® barrier bottles
About LOG:
For the last 50 years, LOG has been serving as a leader in the global pharmaceutical packaging industry, delivering innovative, highly engineered, active, and passive packaging solutions from its manufacturing sites in Israel and Hungary.
LOG adheres to the strictest quality manufacturing standards and is fully committed to compliance with GMP standards for primary packaging. The quality management systems (QMS) of both manufacturing sites are ISO 9001 and ISO 15378 certified.
Stay tuned on new pharma packaging innovations LOG will launch during 2021, signing up for our newsletter and by following us on LinkedIn.
Tags: Barrier Packaging, Drug Formulations, Multilayer bottles, OxyBlock®, Pharma Packaging

